Is It Really Non-Comedogenic? What the Label Doesn’t Tell You

Published on August 21, 2025
In this article
- What “Comedogenic” Actually Means
- The Problem with the Comedogenic Scale
- Why Labels Can't be Trusted
- So when it comes to choosing a skincare routine, how can we avoid triggering acne?
- References
What “Comedogenic” Actually Means
The term comedogenic refers to a substance’s potential to contribute to the formation of comedones — clogged pores that appear as blackheads (open comedones) or whiteheads (closed comedones). These form when the pilosebaceous unit becomes blocked by sebum and keratin, disrupting normal follicular shedding and oil flow [1].
While comedones are the starting point of all acne lesions [1], not all breakouts are simply the result of pore blockage. Other factors — like inflammation within the follicular wall, skin barrier dysfunction, or microbial triggers — can worsen or prolong acne beyond the initial comedone stage [2,3].
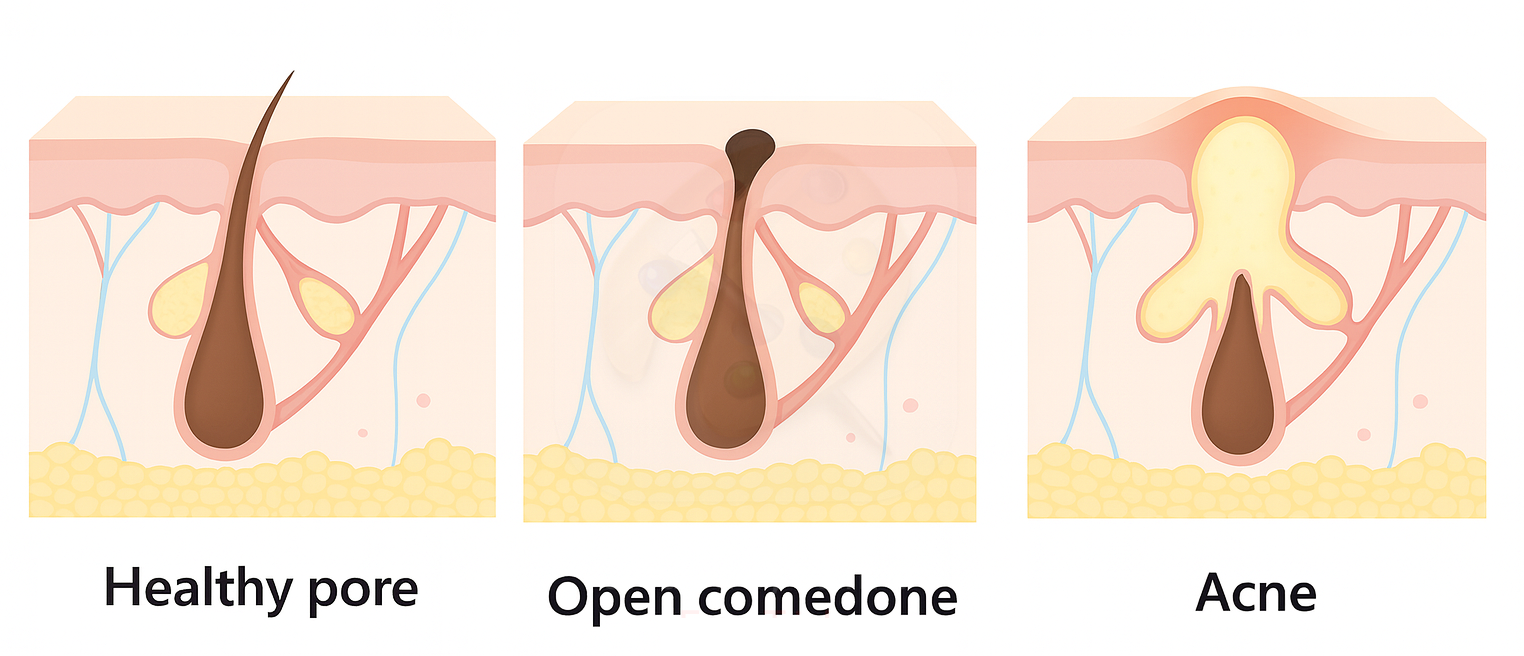
That's why even if a product is labeled non-comedogenic, it can still trigger acne by disrupting the skin barrier or altering the microbiome — pathways that worsen inflammation without necessarily clogging pores [2,3].
For those dealing with acne, it's understandable to seek out products labeled “non-comedogenic” — a term that implies safety from clogged pores. The term traces back to the concept of comedogenic ratings. In an attempt to quantify which ingredients are more likely to clog pores, researchers developed a numerical scale — the so-called comedogenicity scale — assigning scores to ingredients based on how likely they are to cause comedones. But while the idea sounds scientific and reassuring, the reality is far more complicated.
The Problem with the Comedogenic Scale
The comedogenicity scale originated from rabbit ear assays. In these tests, ingredients — often neat or highly concentrated — are applied to rabbit ears to see if comedones form [4]. But the rabbit model is highly sensitive and prone to false positives; many substances flagged in rabbits don’t reliably cause comedones in humans [4,5].
Even human testing of comedogenicity remains imperfect. For example, Mills & Kligman (1982) demonstrated that ingredients flagged as comedogenic in rabbit ears can indeed cause comedones in humans—but when applied under occlusion and in concentrated form using an upper-back patch method [5]. This controlled setting differs dramatically from real-world use, making it questionable whether those results translate to everyday product application on exposed facial skin.
Moreover, comedogenicity isn’t intrinsic to individual ingredients — it depends heavily on formulation. Draelos & DiNardo’s reevaluation found that finished products containing comedogenic ingredients don’t necessarily produce comedones, showing that dose, vehicle, and interactions matter more than a single ingredient [6].
Some critics even question the relevance of the rabbit model entirely. Frank noted that rabbit results often don't correlate to acne development in humans, emphasizing the need for human-based validation [7].
Why Labels Can't be Trusted
“Non-comedogenic” gives the impression of scientific credibility — as if the product has passed a defined test and is guaranteed not to clog pores. But there is no standardized test, no regulatory definition, and no independent verification behind the label. Brands can use the term based on old ingredient ratings, internal evaluations, or simply marketing strategy. No authority oversees how the claim is applied — and no consistency is guaranteed in what it actually means.
Comedogenicity isn’t a fixed trait — it depends on how a product is formulated, who’s using it, and under what conditions.
Context Matters:
-
Concentration matters. Classic comedogenicity assays often applied undiluted or highly concentrated ingredients. Results at those doses/conditions don’t necessarily predict what happens at lower, in-product use levels [8].
-
The vehicle changes everything. Comedogenicity of an ingredient can vary depending on the solvent of the formulation. It might vary drastically depending of it is in an oil, cream, gel, etc. [4]
-
Skin type and environment play a role. Higher sebum output (oily/combination skin) and hot, humid environments are associated with greater acne prevalence and flares; heat also increases sebum excretion [9].
-
Formulation synergy matters. Some ingredient combinations amplify the pore-blocking effect of certain ingredients, becoming more comedogenic than each of the individual ingredients [4].
And last but not least, ingredient lists are only part of the picture - personal sensitivity is unpredictable.
Pin it!

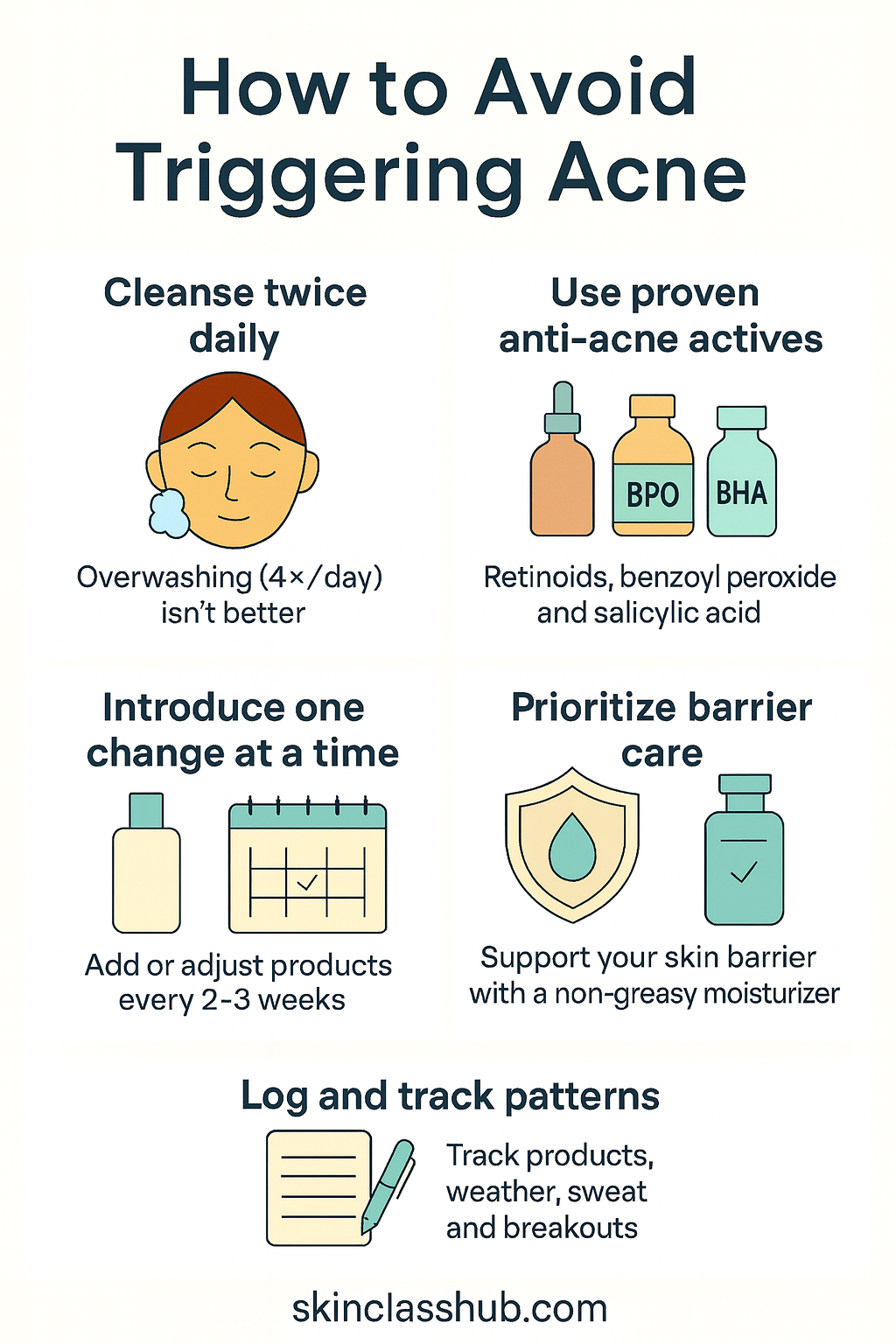
So when it comes to choosing a skincare routine, how can we avoid triggering acne?
-
Cleanse twice daily.
Twice-daily washing improved non-inflammatory lesions vs once daily in an randomized controlled trial; overwashing (4×/day) wasn’t better. [10] -
Use proven anti-acne actives.
Retinoids, benzoyl peroxide, and salicylic acid (BHA 0.5–2%) are evidence-based options for comedonal/mild acne; build tolerance slowly and pair with barrier care [11]. -
Prioritize barrier care.
Use products that support barrier function instead of stripping it; barrier repair is part of acne management. [2] -
Introduce one change at a time. Add/adjust products no more than every 2–3 weeks so you can spot culprits.
-
Log what you use and the context.
Note product, date, weather, sweat/occlusion, and breakouts. Patterns often track with environment and oiliness. [9]
Pin it!
References
-
Sutaria, A. H., Masood, S., Saleh, H. M., et al. (2023). Acne vulgaris. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Updated August 17, 2023.
Acne Vulgaris - StatPearls - NCBI Bookshelf -
Deng, Y. et al (2024). Skin Barrier Dysfunction in Acne Vulgaris: Pathogenesis and Therapeutic Approaches. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 30, e945336. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11650898/
-
Kemény, L., Degovics, D., Szabó, K. (2025). Is there a place for biologics in acne? American Journal of Clinical Dermatology. https://link.springer.com/article/10.1007/s40257-025-00954-8
-
Fulton, J. E. Jr. (1989). Comedogenicity and irritancy of commonly used ingredients in skin care products. Journal of the Society of Cosmetic Chemists, 40(6), 321–333. https://insolitbeauty.com/documentacion/Comedogenicidad%20e%20irritacion%20de%20los%20ingredientes%20de%20uso%20comun%20en%20productos%20para%20el%20cuidado%20de%20la%20piel.pdf
-
Mills, O. H. Jr., Kligman, A. M.(1982). A human model for assessing comedogenic substances. Archives of Dermatology, 118(11), 903–905.
A human model for assessing comedogenic substances - PubMed -
Draelos, Z.D., & DiNardo, J.C. (2006). A re-evaluation of the comedogenicity concept. Journal of the American Academy of Dermatology, 54(3), 507–512. https://www.sciencedirect.com/science/article/abs/pii/S0190962205046001
-
Frank, S.B. (1982). Is the rabbit ear model, in its present state, prophetic of acnegenicity? *Journal of the American Academy of Dermatology, 6(3), 373–377. https://www.sciencedirect.com/science/article/abs/pii/S0190962282700325
-
Waranuch, N., Wisutthathum, S., Tuanthai, S., et al. (2021). Safety assessment on comedogenicity of dermatological products containing d-alpha tocopheryl acetate in Asian subjects: A double-blind randomized controlled trial. Contemporary Clinical Trials Communications, 23, 100834 https://pmc.ncbi.nlm.nih.gov/articles/PMC8387765
-
Dréno, B., Bettoli, V., Araviiskaia, E., et al. (2018). The influence of exposome on acne. Journal of the European Academy of Dermatology and Venereology, 32(5), 812–819. https://pmc.ncbi.nlm.nih.gov/articles/PMC5947266/
-
Choi, J. M., Lew, V. K., Kimball, A. B. (2006). A single-blinded, randomized, controlled clinical trial evaluating the effect of face washing on acne vulgaris. Pediatric Dermatology, 23(5), 421–427. https://pubmed.ncbi.nlm.nih.gov/17014635/
-
Reynolds, R. V., Yeung, H., Cheng, C. E., et al. (2024). Guidelines of care for the management of acne vulgaris. Journal of the American Academy of Dermatology, 90(5), 1006.e1–1006.e30. https://pubmed.ncbi.nlm.nih.gov/38300170/